
At CD BioSciences, we specialize in delivering comprehensive Diagnostics Development Solutions for Human Herpesvirus (HHV), supporting clients in the discovery, validation, and assessment of diagnostic biomarkers. Our cutting-edge solutions cater to pharmaceutical companies, biotechnology firms, and research institutions, focusing on the preclinical phase to ensure precision and efficacy in your diagnostic research and therapeutic development.
Our diagnostics services are structured to provide insights into HHV pathogenesis, improve early detection of HHV-associated diseases, and enable the development of targeted therapeutic interventions. We offer a full suite of services that help you progress from biomarker discovery to assay development and validation, addressing the growing need for effective diagnostics in HHV-related research.
Biomarker Discovery & Validation
The identification and validation of reliable biomarkers are crucial for understanding the complex dynamics of HHV infections. Our team utilizes advanced technologies and methodologies to discover and validate biomarkers that can aid in the early detection of HHV-related diseases such as Kaposi's Sarcoma, Herpes Simplex Virus (HSV) infections, and Epstein-Barr Virus (EBV)-related malignancies.
Our biomarker discovery services cover:
- Proteomics: Using mass spectrometry and antibody-based assays, we identify novel biomarkers that can serve as diagnostic indicators for HHV-related conditions.
- Genomics: Leveraging next-generation sequencing (NGS), we detect genetic alterations in host cells caused by HHV infection, enabling the identification of candidate biomarkers for disease progression and therapeutic response.
- Immunology: Our immunoassay development capabilities allow us to pinpoint immune responses to HHV infections, facilitating the discovery of immune biomarkers for patient stratification and personalized treatment.
Once biomarkers are identified, we carry out rigorous validation studies to ensure their reliability, specificity, and sensitivity in detecting HHV infection across various patient populations.
Assay Development Support
Developing accurate and reproducible diagnostic assays is vital for translating biomarkers into usable tools for clinical and preclinical settings. At CD BioSciences, we offer extensive support in assay development, ensuring that your diagnostic tests meet industry standards and provide actionable results.
Our assay development services include:
We tailor assay development to your specific needs, ensuring that your assays are optimized for the intended application, whether for early detection, disease monitoring, or treatment response.
Validation of Diagnostic Tools
Once the biomarker and assay systems are in place, we provide comprehensive validation support. This phase ensures that the diagnostic tools are clinically relevant, reliable, and suitable for widespread use.
Our validation services include:
- Analytical Validation: Confirming the sensitivity, specificity, and reproducibility of diagnostic tests.
- Clinical Validation: Testing the diagnostic tool in clinical settings, comparing its performance with existing methods to assess its diagnostic accuracy and utility.
- Regulatory Support: We assist clients in navigating regulatory pathways, providing support for obtaining approvals from health authorities like the FDA and EMA for diagnostic kits intended for clinical use.
End-to-End Support for HHV Diagnostic Solutions
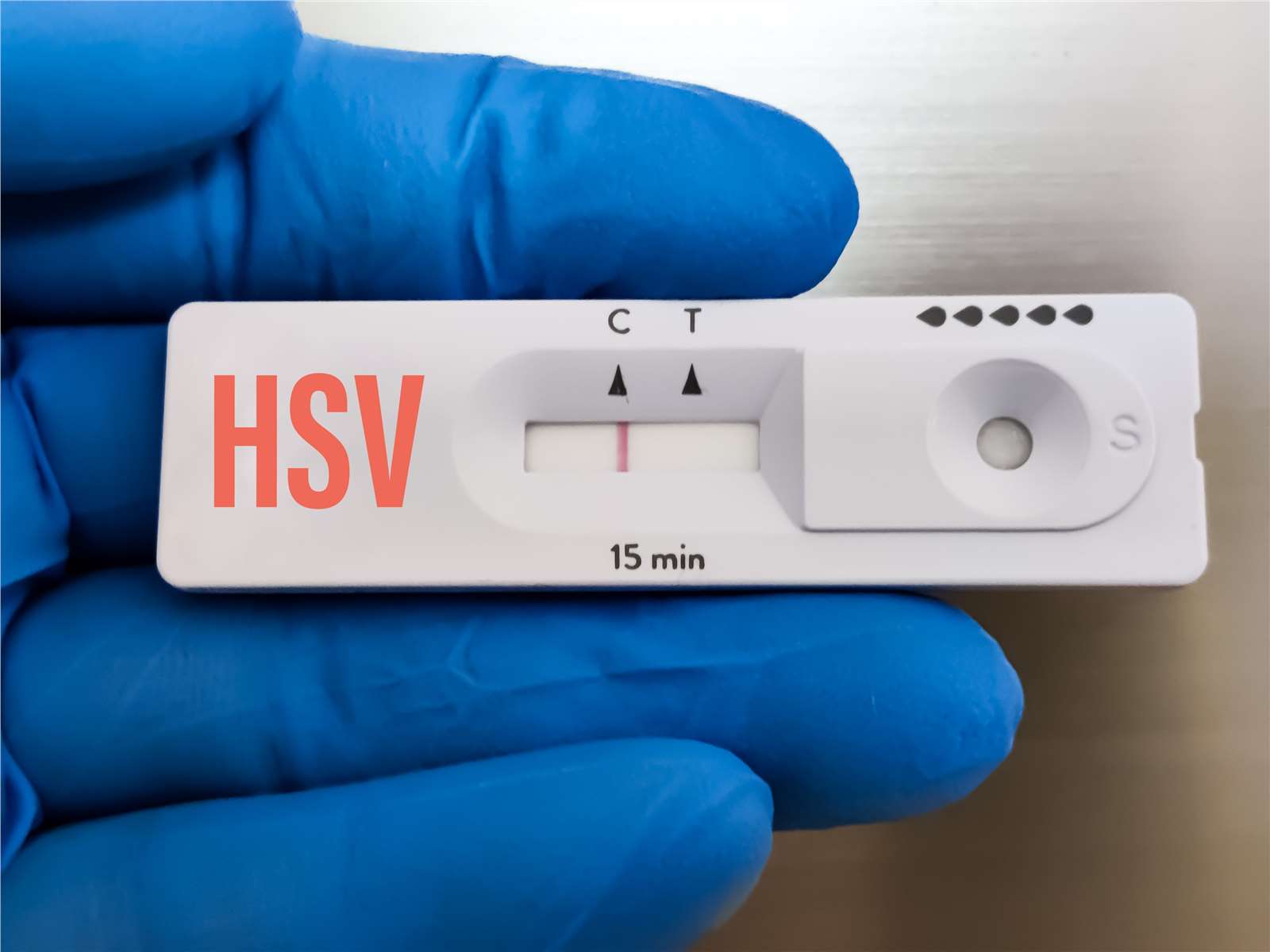
We provide end-to-end support throughout the diagnostics development process, from initial biomarker discovery to the final validation of diagnostic tools. Our services are designed to accelerate your research and development timelines, ensuring that you have access to the most advanced technologies and methodologies.
We collaborate closely with our clients, offering tailored solutions based on their specific needs and research objectives. Whether you are developing novel diagnostic tests for latent or active HHV infections or need comprehensive biomarker validation for new clinical trials, CD BioSciences is committed to helping you succeed.
Why Choose CD BioSciences?
- Expertise: Our team consists of seasoned professionals with expertise in virology, immunology, and diagnostics, ensuring that you receive scientifically rigorous and innovative solutions.
- Cutting-edge Technology: We utilize state-of-the-art technologies such as NGS, mass spectrometry, and advanced immunoassay systems to ensure that our diagnostics solutions are at the forefront of the industry.
- Customized Solutions: We understand that every project is unique, and we offer highly flexible and personalized services tailored to your specific research and diagnostic needs.
- Regulatory Knowledge: Our team is well-versed in the regulatory requirements for diagnostic tools, and we provide valuable support to help you navigate the approval process efficiently.
In conclusion, CD BioSciences is dedicated to supporting your research and diagnostic development efforts in the field of Human Herpesvirus (HHV). With our comprehensive suite of services—from biomarker discovery to assay development and validation—we help you create accurate, reliable, and clinically useful diagnostic solutions for HHV-related diseases. Trust us to provide you with the expertise and support necessary to bring your diagnostic innovations to life.
References
- Mocarski, E. S., et al. (2013). "Herpesviruses." Nature Reviews Disease Primers, 1, 15009.
- Furuta, R., et al. (2018). "Human herpesvirus and its diagnostic markers." Journal of Clinical Microbiology, 56(10), e01425-18.
- Ha, S. J., et al. (2020). "Advances in diagnostics for Herpes simplex virus infections." Diagnostic Microbiology and Infectious Disease, 98(4), 115159.