
Welcome to the CD BioSciences HHV Knowledge Center, a dedicated resource for scientists, researchers, and developers in the biotechnology and pharmaceutical industries. This center provides a comprehensive overview of the Human Herpesvirus (HHV) family, from fundamental biology to the latest advancements in therapeutic research. Our mission is to empower the scientific community by sharing in-depth knowledge and offering specialized, pre-clinical research solutions to tackle the challenges posed by these complex pathogens.
The HHV Family
The family Herpesviridae comprises a large group of double-stranded DNA viruses found in a wide range of animal species. To date, nine human herpesviruses have been identified, each responsible for a variety of diseases ranging from mild mucocutaneous lesions to severe neurological disorders and cancers.
- Introduction to Herpesviridae
Human herpesviruses are ubiquitous, with a significant portion of the global population being seropositive for multiple members of the family. Their defining biological characteristic is the ability to establish a lifelong, latent infection in their hosts. Following a primary infection, the virus can enter a dormant state within specific cell types, only to reactivate later under certain conditions, such as immunosuppression, stress, or hormonal changes. This cycle of latency and reactivation presents significant challenges for both treatment and vaccine development.
- Classification: Alpha-, Beta-, and Gamma-herpesviruses
The HHV family is categorized into three subfamilies—Alphaherpesvirinae, Betaherpesvirinae, and Gammaherpesvirinae—based on their biological properties, including host range, replication cycle duration, and site of latency.
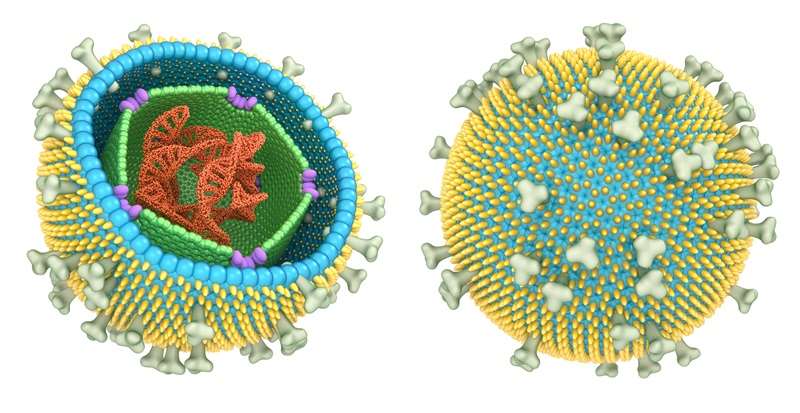
Alpha-herpesviruses (α-HHVs)
Characterized by a rapid replication cycle, broad host range, and the ability to establish latency primarily in sensory neurons. This subfamily includes Herpes Simplex Virus 1 & 2 (HSV-1, HSV-2) and Varicella-Zoster Virus (VZV).
Beta-herpesviruses (β-HHVs)
These viruses have a slower, more restricted replication cycle. They establish latency in various cell types, including myeloid progenitor cells, lymphocytes, and endothelial cells. Members include Cytomegalovirus (CMV), Human Herpesvirus 6A & 6B (HHV-6A, HHV-6B), and Human Herpesvirus 7 (HHV-7).
Gamma-herpesviruses (γ-HHVs)
These viruses are lymphotropic, establishing latency specifically within lymphoid cells (B or T lymphocytes). They are distinguished by their ability to induce cell proliferation and are associated with several types of cancer. This subfamily includes Epstein-Barr Virus (EBV) and Kaposi's Sarcoma-associated Herpesvirus (KSHV/HHV-8).
- Common Features: Structure, Genome, and Replication Cycle
Despite their diversity, all herpesviruses share a common virion architecture. The structure consists of a large, linear double-stranded DNA genome enclosed within an icosahedral capsid. This capsid is surrounded by an amorphous protein layer known as the tegument, which is, in turn, enveloped by a lipid bilayer studded with viral glycoproteins.
The replication cycle begins with the virus binding to host cell receptors, followed by fusion of the viral envelope with the cell membrane. The capsid is then transported to the nucleus, where the viral DNA is released and circularizes. Gene expression occurs in a tightly regulated cascade (immediate-early, early, and late genes), leading to DNA replication, assembly of new virions, and finally, egress from the host cell. Understanding the molecular details of this process is critical for identifying novel antiviral targets, a key focus of our pre-clinical research services.
- The Hallmarks of Latency and Reactivation
Latency is the cornerstone of HHV biology. During this phase, the viral genome persists as a stable, circular episome in the nucleus of the latently infected cell with very limited gene expression. This allows the virus to evade the host immune system. Reactivation is a complex process triggered by various stimuli, leading to the re-initiation of the lytic replication cycle and the production of infectious virions. The molecular switches that govern the latency-reactivation cycle are a major area of research, as blocking reactivation is a key therapeutic goal.
Alpha-herpesviruses
Herpes Simplex Virus 1 & 2 (HSV-1, HSV-2)
HSV-1 is traditionally associated with orofacial lesions ("cold sores"), while HSV-2 is the primary cause of genital herpes. However, this distinction is becoming less clear. Both are significant causes of morbidity and can lead to severe conditions like keratitis (ocular disease) and encephalitis (neurological disease).
Varicella-Zoster Virus (VZV)
VZV is the causative agent of varicella (chickenpox) upon primary infection. It then establishes latency in cranial nerve and dorsal root ganglia. Reactivation, typically occurring later in life, results in herpes zoster (shingles), a painful skin rash that can lead to chronic postherpetic neuralgia.
Beta-herpesviruses
Cytomegalovirus (CMV)
CMV infection is widespread and usually asymptomatic in healthy individuals. However, it is a major cause of disease in immunocompromised individuals, such as transplant recipients and those with HIV/AIDS. Congenital CMV infection is also a leading cause of birth defects, including hearing loss and neurodevelopmental delays. The development of effective CMV antivirals and vaccines remains a high-priority research goal.
Human Herpesvirus 6 (HHV-6A, HHV-6B)
HHV-6B is the primary cause of exanthem subitum (roseola infantum), a common childhood illness. The clinical roles of HHV-6A are less defined but have been linked to neurological conditions like multiple sclerosis. Both viruses can reactivate in immunocompromised hosts, causing significant complications.
Human Herpesvirus 7 (HHV-7)
Closely related to HHV-6, HHV-7 is also commonly acquired in childhood and is sometimes associated with roseola. Its latency in T-cells and broader clinical significance are areas of active investigation.
Gamma-herpesviruses
Epstein-Barr Virus (EBV)
EBV is one of the most common human viruses, best known for causing infectious mononucleosis. As a lymphotropic virus, it has potent transforming capabilities and is etiologically linked to several cancers, including Burkitt's lymphoma, Hodgkin's lymphoma, nasopharyngeal carcinoma, and certain gastric cancers. Studying the mechanisms of EBV-induced oncogenesis is critical for developing targeted therapies.
Kaposi's Sarcoma-associated Herpesvirus (KSHV/HHV-8)
KSHV is the causative agent of Kaposi's sarcoma, a malignancy commonly seen in AIDS patients. It is also linked to two other lymphoproliferative disorders: primary effusion lymphoma and multicentric Castleman disease. Research into KSHV focuses on understanding how its viral oncogenes subvert host cell control. CD BioSciences offers robust in vitro models and custom assays for screening compounds against these oncogenic viruses.
HHV-Associated Diseases
The clinical spectrum of HHV infections is vast.
- Cutaneous & Mucosal Diseases: The most common manifestations, from HSV-induced cold sores and genital herpes to VZV-induced shingles.
- Ocular Diseases: HSV, VZV, and CMV can all cause serious eye infections, such as keratitis and retinitis, which can lead to vision loss.
- Neurological Diseases: Several HHVs are neurotropic. They can cause severe conditions like encephalitis, meningitis, and radiculopathy.
- Diseases in Immunocompromised Hosts: In individuals with weakened immune systems (e.g., organ transplant recipients, cancer patients), HHV reactivation (especially CMV, EBV, and VZV) can be life-threatening, leading to pneumonia, hepatitis, and disseminated disease.
- Cancers & Proliferative Disorders: The oncogenic potential of gamma-herpesviruses EBV and KSHV is a major global health concern, driving extensive research into viral oncogenesis and immunotherapy.
Antiviral Drug R&D
The development of antiviral drugs is a cornerstone of managing HHV diseases, particularly for immunocompromised patients and those with severe infections.
- Key Drug Targets

The viral DNA polymerase has been the most successful drug target to date, with nucleoside analogs like acyclovir, ganciclovir, and their derivatives forming the backbone of current therapy. However, the emergence of drug-resistant strains has driven the search for novel targets. Promising new targets under investigation include the helicase-primase complex, which is essential for unwinding viral DNA, and the viral terminase complex, which packages the genome into new capsids.
- Therapeutic Strategies
Current research extends beyond small molecule inhibitors. Strategies include:
Monoclonal Antibodies: For prophylaxis, especially against CMV in high-risk transplant settings.
Therapeutic Vaccines: Aimed at boosting T-cell responses to control latent infection and prevent reactivation.
Novel Mechanisms: Development of drugs that inhibit viral entry, nuclear egress, or modulate the host response.
- R&D Landscape
The antiviral R&D landscape is focused on overcoming the limitations of current drugs, such as toxicity and resistance. There is a critical need for new agents with novel mechanisms of action, particularly for beta- and gamma-herpesviruses, for which therapeutic options are limited. High-throughput screening (HTS) campaigns, mechanism-of-action (MoA) studies, and the development of more predictive pre-clinical models are essential for progress. CD BioSciences provides comprehensive platforms to support every stage of this pre-clinical drug discovery pipeline, from target validation to lead optimization.
Vaccine R&D
A vaccine remains the ultimate goal for preventing HHV-associated diseases.
- Vaccine Types
Vaccine development efforts encompass several types:
Prophylactic Vaccines: Designed to prevent initial infection.
Therapeutic Vaccines: Aimed at controlling or clearing established latent infection by stimulating cellular immunity.
Congenital Disease Vaccines: Specifically focused on preventing maternal-fetal transmission, a major goal for CMV.
- Vaccine Platforms & Technologies
The only licensed HHV vaccine is the live-attenuated VZV vaccine for chickenpox and shingles, which is highly effective. For other HHVs like HSV and CMV, development has been challenging. Modern platforms are now being leveraged to overcome these hurdles:
Subunit Vaccines: Using specific viral glycoproteins (e.g., gB for CMV, gD for HSV) combined with powerful adjuvants.
Nucleic Acid Vaccines (mRNA, DNA): This technology allows for rapid development and can elicit both antibody and T-cell responses.
Viral Vector Vaccines: Using vectors like adenovirus or modified vaccinia ankara (MVA) to deliver HHV antigens.
- Development Landscape
The HHV vaccine landscape is dynamic and challenging. Past failures of promising candidates have highlighted the complexity of generating protective immunity against these viruses. Key challenges include identifying the optimal antigens and overcoming viral immune evasion mechanisms. Despite this, significant progress is being made, particularly with adjuvant technology and mRNA platforms, offering new hope for effective vaccines against CMV, EBV, and HSV. Our team provides support for pre-clinical vaccine evaluation, including immunogenicity testing and challenge model development.
Disclaimer: The products and services offered by CD BioSciences are for Research Use Only (RUO) and are not intended for clinical or diagnostic use. They are not for use in humans or animals for therapeutic purposes.
References
- Whitley, R. J., & Roizman, B. (2001). Herpes Simplex Virus Infections. The Lancet, 357(9267), 1513–1518.
- Arvin, A., Campadelli-Fiume, G., Mocarski, E., et al. (Eds.). (2007). Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press.
- Cohen, J. I. (2020). Herpesvirus Latency. Journal of Clinical Investigation, 130(7), 3361–3369.
- Longnecker, R., Neipel, F., & Cliffe, A. (2020). The role of human herpesviruses in cancer. Cancer, 126(18), 4103-4122.
- Griffiths, P. D. (2018). CMV as a drug and vaccine target. Journal of Antimicrobial Chemotherapy, 73(suppl_1), i39-i44.
- Heineman, T. C., & Cunningham, A. L. (2019). A future for herpes simplex virus vaccines. The Lancet Infectious Diseases, 19(10), 1046-1048.