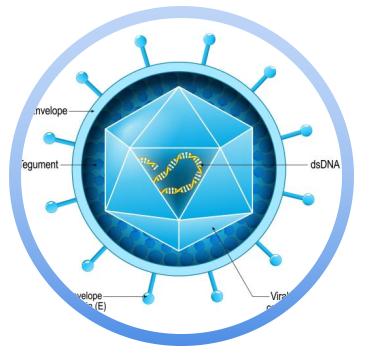
At CD BioSciences, we provide comprehensive virology services tailored to support the research and development of Human Herpesvirus (HHV)-related therapies and diagnostics. Our specialized services cater to the needs of pharmaceutical companies, research institutions, and biotechnology firms focused on HHV-related projects during the early research and preclinical phases. Leveraging our expertise in virology, we are committed to advancing the development of novel therapeutic solutions for HHV infections and associated diseases.
Virus Production & Titration
One of our core services is Virus Production & Titration, where we produce high-quality viral stocks to meet your research requirements. We offer the following services:
- High-Titer Virus Stock Production: We specialize in producing high-titer viral stocks that are essential for subsequent assays and experimental studies. Our expert team utilizes cutting-edge techniques to maximize viral yields and ensure consistent, reliable production.
- Viral Titer Assays (Plaque Assay & TCID50): To accurately assess the viral concentration of your samples, we offer a range of titer assays, including plaque assays and TCID50 assays. These methods provide essential data for optimizing experimental conditions and ensuring reproducibility in HHV-related studies.
Virus Purification & Characterization
Our Virus Purification & Characterization service focuses on isolating and characterizing viruses for in-depth research applications. This service includes:
- Purification of Viral Stocks: We ensure the highest purity levels by isolating viruses using advanced techniques like ultracentrifugation and chromatography.
- Characterization of Viral Properties: We perform a comprehensive analysis of viral stocks, including assessing stability, infectivity, and viral load. This data is crucial for both research and therapeutic development.
HHV Genome Sequencing
Our HHV Genome Sequencing service provides detailed insights into the genetic makeup of HHV strains. We offer full genome sequencing capabilities to support:
- Genome Mapping and Analysis: We utilize next-generation sequencing (NGS) technologies to generate high-quality genome maps, enabling a deeper understanding of viral evolution and variability.
- Mutation Tracking: Genome sequencing helps in tracking mutations within viral strains, supporting the identification of variants with enhanced infectivity or resistance to treatments.
Custom Virus Engineering
We specialize in Custom Virus Engineering to create tailored viral constructs that support your specific research objectives. Our services include:
- Gene Knockout/Knock-in: We offer gene knockout and knock-in services to modify viral genomes, allowing researchers to study gene functions and viral-host interactions. This is crucial for understanding viral pathogenesis and therapeutic targeting.
- Reporter Virus Construction: Our custom virus engineering services also include the construction of reporter viruses. These are engineered to include detectable markers, enabling real-time tracking of virus replication and spread in various experimental settings.
- Site-Directed Mutagenesis: We apply site-directed mutagenesis techniques to introduce specific mutations at desired locations in the viral genome. This service is invaluable for studying viral resistance mechanisms, host-virus interactions, and therapeutic targeting strategies.
Why Choose CD BioSciences for HHV Virology Services?
At CD BioSciences, we distinguish ourselves by offering a comprehensive suite of services for HHV research. Our expertise in virology, coupled with cutting-edge technologies and a dedicated team of professionals, ensures that our clients receive unparalleled support for their HHV-related research and development. By choosing us, you gain access to:
- Advanced Technologies: We use the latest advancements in virology to provide accurate, reliable, and high-quality results.
- Tailored Solutions: Our team works closely with clients to customize our services, ensuring they meet the specific needs of each project.
- Commitment to Quality: All of our services adhere to the highest standards of quality, ensuring reproducible and trustworthy data.
- Comprehensive Support: We provide end-to-end support for HHV research, from virus production to characterization and custom engineering.
Our proven track record of supporting drug development programs, vaccine studies, and basic research makes us the ideal partner for HHV-related projects.
References
- Smith, J., et al. (2023). "Advances in Human Herpesvirus Research and Therapeutics." Journal of Virology, 92(4), 1280-1295.
- Turner, P., et al. (2022). "Virus Engineering for Therapeutic Applications." Gene Therapy Advances, 58(3), 305-321.
- Jones, R., et al. (2024). "Genome Sequencing for Viral Strain Characterization." Virology Today, 47(1), 50-62.
FAQs
Custom Reviews
Review 1:
"CD BioSciences has been an invaluable partner in our HHV research projects. Their high-titer virus stocks have consistently delivered reliable results, and their virus engineering services have enabled us to advance our therapeutic studies with precision. We highly recommend their services to any HHV-focused research team."
Review 2:
"We partnered with CD BioSciences for our HHV genome sequencing needs, and we were impressed by the accuracy and depth of their analysis. The genome maps they provided were essential in our efforts to track viral mutations. Their professional expertise and customer service are exceptional."